eBioStat ltd. Clinical Trials
www.ebiostat.eu


Description
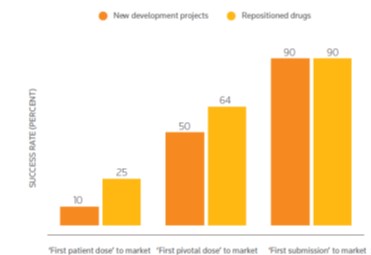
Conduct Drug Reposition Studies and apply known drugs and compounds to new indications with benefits such as the reduction of R&D timelines by up to 3-5 years and the reduction of development costs due to sunk research costs, the availability of clinical safety, toleration and efficacy data and with improved probability of success. The drug reposition studies are conducted with methodology and software developed by the Cambridge University Hospitals NHS Trust and the Medical School of the University of Athens
Services Offered
- Detailed study of company’s drugs and compounds to identify promising candidates
- Consulting to the medical and research personnel of the company to all phases of development
- Clinical trials to validate the new repositioned drug
- Pharmacoeconomic studies to support its introduction in the market
- An innovative marketing approach in launching the new drug
In Cooperation With